A Buffer Solution Comprises Which of the Following
Is HClO4 and NaClO4 a buffer. The bthe solution buffer.
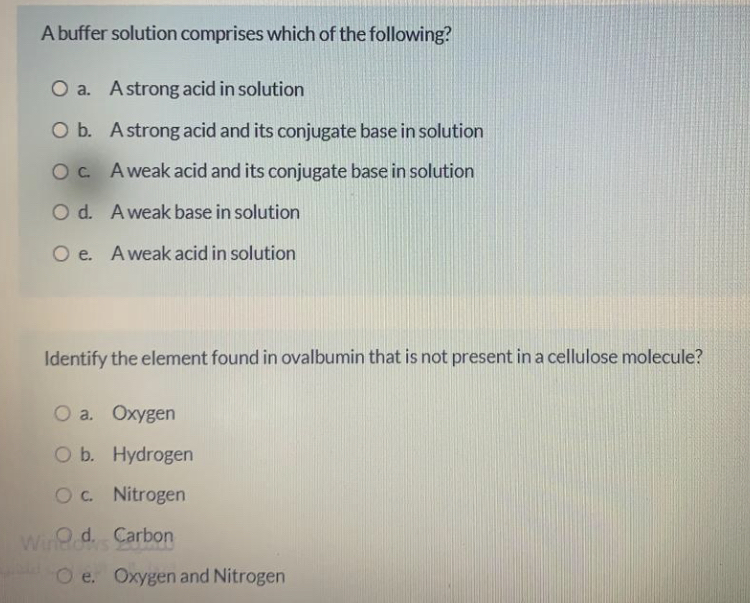
Answered A Buffer Solution Comprises Which Of Bartleby
A strong acid in solution P Flag question O c.

. A solution that doesnt resist significant changes in pH upon addition of a small amount of a strong acid or base. A buffer solution can be made by mixing a weak acid with one of its salts OR mixing a weak base with one of its salts. Hence solution of acetic acid and sodium acetate is a Buffers solution.
B strongish acid weak base. Lewis Which of the following type of electrode used in pH metre. Astrong acid in solution Ob.
AA weak acid in solution. A buffer solution contains t what pH don contains ethanoic acid and its conjugate base. Explanation- A buffer solution is a.
59The electrolyte solution within the glass electrode reference of the pH meter is a saturated KCl b. Buffers can be made from three combinations. A weak acid in solution O b.
A more technical way of saying this is that a buffer solution consists of a mixture of a weak acid and its conjugate base OR a weak base and its conjugate acid. A buffer is the combination of a weak acid or base and a salt of that weak acid or base. A buffer solution is an aqueous solution of weak acid and its conjugate baseIt can also be a mixture of weak base and its conjugate base.
What can you conclude about the concentrations of the components of the buffer. CA weak base in solution. D weak acid and a half equiv strong base.
The Ka of HA 10 x 10 -4. A weak base and. So looking at your list a has strongish acid strong base.
A buffer solution comprises which of the following a. A buffer has a pH of 485 and contains formic acid and potassium formate. In the given options only option-A has weak acid CH 3 COOH and its conjugate base CH 3 COONa Option-B.
Question 5 A buffer solution comprises which of the following. Not yet answered Marked out of 150 O a. The ph of a liquid solution is a measure of a.
View the full answer. Buffer solution This is a mixture that minimises pH changes on addition of small amounts of acid or base A weak acid is used and its conjugate base formed by adding weak acid salt or neutralising the acid by adding aqueous alkali. A solution made by mixing 100 mL of 0100 M HClO and 50 mL of 0100 M HCl IS NOT a buffer.
Weak acid conjugate base Acidic buffer Weak base conjugate acid Basic buffer By producing alternative components this mixture preserves pH homeostasis in the face of any pH shift. Among the given options N H 4 O H N H 4 C l is the only mixture which consists of a mixture of a weak base and its conjugated salt and thus has a p H greater than 7. The pKa of ethanoic acid is 474.
DA weak acid and its conjugate base in solution. The pH of a liquid solution is a measure of a dissolved salt content b hydrogen ion activity c hydroxyl ion molarity d electrical conductivity Answer. A buffer is defined as a substance which is able to resist changes in pH of a solutionIt usually comprises of the mixture of a weak acid with its conjugate base or a weak base with its conjugate acid.
The maximum buffer capacity is achieved when pH pKa or in equivalent terms where H3O Ka. A A weak acid in solution b A strong acid in solution c A weak base in solution d A weak acid and its conjugate base in solution. Aweak acid and its conjugate base in solution Oc Aweak acid in solution O d.
At what pH does the solution buffer. At what pH does the solution buffer. 2 A buffer solution could consist of equal concentrations of perchloric acid HClO4 and sodium perchlorate.
Aweak base jn solution O e. BA strong acid in solution. Answer- The correct option is d.
The reaction between HNO₃ and NaF can be deduced below. D HClO4 is a strong acid and hence this is not a buffer system. Which of the following solutions result in a buffer.
C weak acid and a half equiv strong base. Here weak acid is HClO and its conjugate base is NaClO. A Buffer solution contains 036 M sodium acetate CH3 COONA And 045 M acetic acid CH3 COOH pKa48 what is the pH of this buffer solution.
Astrong acid and its conjugate base in solution. The p H value of a basic buffer is always greater than 7. HNO₃ NaF HF NaNO₃.
A buffer acts to resist GROSS changes in pH. Maximum buffer capacity. 3 A buffer solution will change only slightly in pH upon addition of small amounts of acid or base.
Science Biochemistry QA Library A buffer solution comprises which of the following. Acid and its conjugate base in c A weak base in solution pka of ethanoic acid is 474 14. The concept of pH was introduced by A.
4 In a buffer solution containing benzoic acid C6H5COOH and sodium benzoate NaC6H5COO the species that reacts with added hydroxide ion is the benzoate ion. Which of these combinations produces a buffer solution. 1 H 3PO 4 and H 2PO 4 2 H 2PO 4 and HPO 4 2 and 3 HPO 4 2 and PO 4 3.
A weak base in solution O d. A buffer is a mixture of a solution a weak acid and its conjugate base or vice versa. The K a of formic acid is 18 x 10 -4.
154 A buffer solution comprises which of the following. A buffer solution is formed when appreciable quantities of a weak acid and its conjugate base are mixed together in aqueous solution. Buffer solutions are obtained when a weak acid is mixed with its conjugate base or a weak base is mixed with its conjugate acid.
Thus a solution by mixing 100 mL of 0100 M HClO and 50 mL of 0100 M NaOH IS a buffer because. A buffer solution contains ethanoic acid and its conjugate base. The definition of a buffer is answer choices a solution that resists significant changes in pH upon addition of a large amount of a strong acid or base.
HClO NaOH NaClO. A 0100 L of 0250 M NH3 0050 L of 0250 M HCl b 0100 L of 0250 M NH3 0100 L of 0250 M HCl c 0100 L of 0250 M NH4Cl 0050 L of 0250 M NaOH d 0100 L of 0250 M NH4Cl 0150 L of 0250 M NaOH Homework Equations M molV The Attempt at a Solution so I got the mol for each. Suppose you have a solution of 03 M HBrO and 03 M BrO.
A buffer comprises Solution a A weak acid in solution b A strong acid in solution comprises which of the following. Multiple choice questions MCQs 1A buffer solution comprises which of the following. A buffer comprises of either.
There are no single components in it such as only weak acid strong acid or weak base. What is the pH of a buffer that is 008 M NaA and 01 HA. What is a Buffer.

Solved 13 A Buffer Comprises Solution A A Weak Acid In Chegg Com
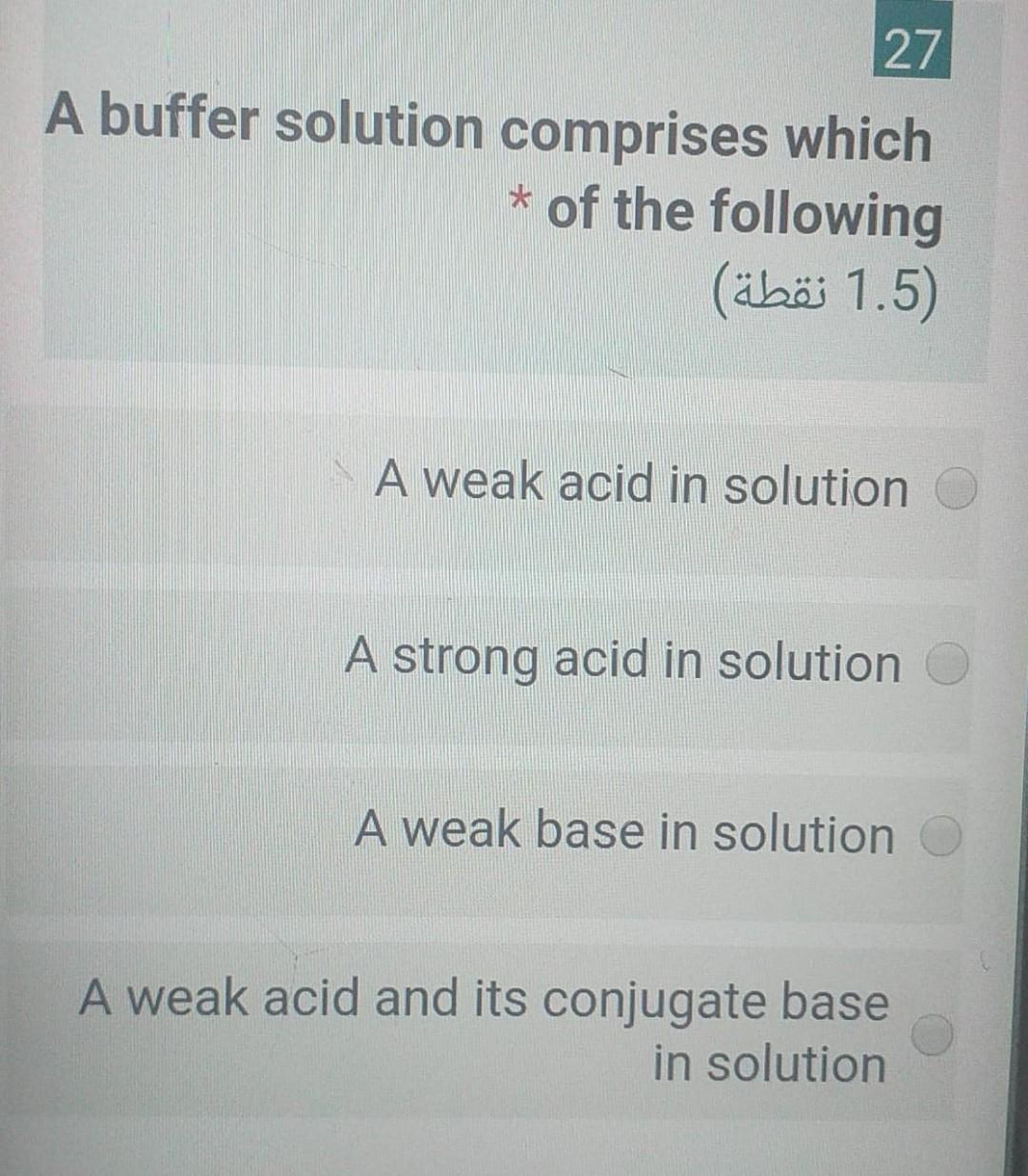
Solved 27 A Buffer Solution Comprises Which Of The Chegg Com

Solved Question 7 A Buffer Solution Comprises Which Of The Chegg Com
0 Response to "A Buffer Solution Comprises Which of the Following"
Post a Comment